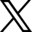You
Cefaly
Sector
e-Health
MedTech
Mobile apps
Challenge
Cefaly is a European-American health products company with a mission to solve the intractable problem of migraine through advanced therapeutic innovations. The name CEFALY is derived from céphalée, the French word for headache, and cephalic, a medical term meaning “related to the head.”
Since 2008, they have developed and improved their proprietary device, CEFALY DUAL, to provide safe and effective drug-free treatment to people with migraine. While the causes of migraine are not yet fully understood, this device acts on the largest and most complex cranial nerve, the trigeminal nerve, which is instrumental in the pain sensation of migraine. CEFALY DUAL is specifically designed to alter pain sensation in the trigeminal nerve via its ophthalmic branch, which runs under the skin of the forehead.
Cefaly’s challenge to LOAD was to create what was simply the world’s best migraine management app. The challenge was met, and LOAD designed and developed a mobile app to better manage patients’ migraine activity and the treatment they use, either to prevent or mitigate each migraine episode.
Our approach
This process started with several secure and sustainable steps by developing a proof of concept of an app with two main directions:
- A migraine management app that can support a community of migraine sufferers;
- Being able to connect to the Cefaly device via Bluetooth (BLE).
This proof of concept was a success and the development of the app was approved, as well as the development of the connected version of the Cefaly device (by Cefaly laboratories).
After the first experience with the prototype and a study of the needs of users and the market, carried out in collaboration with Verhaert Masters in Innovation, began the design and development of an MVP, named Cece Migraine Management App.
Thus, an initial Cece V1 was developed with the goal of creating a Cefaly sufferers community to help them manage their migraine attacks, keep track of their symptoms, triggers, and treatments (drug or non-drug), and help them create a history of events and identify any patterns associated with the migraine attacks.
While Cece V1 was being developed and launched, the connected version was being developed in the Cefaly labs and future versions of the app were being designed and developed. So stay tuned, Cefaly will have news soon!
Solution
The main guidelines for V1 were to allow Cece users to:
- Identify their migraine triggers;
- To see how their attacks develop;
- Identify trends in their migraine attacks;
- Track the effectiveness of their treatment;
- Keep a detailed migraine diary.
And the key CeCe features identified were:
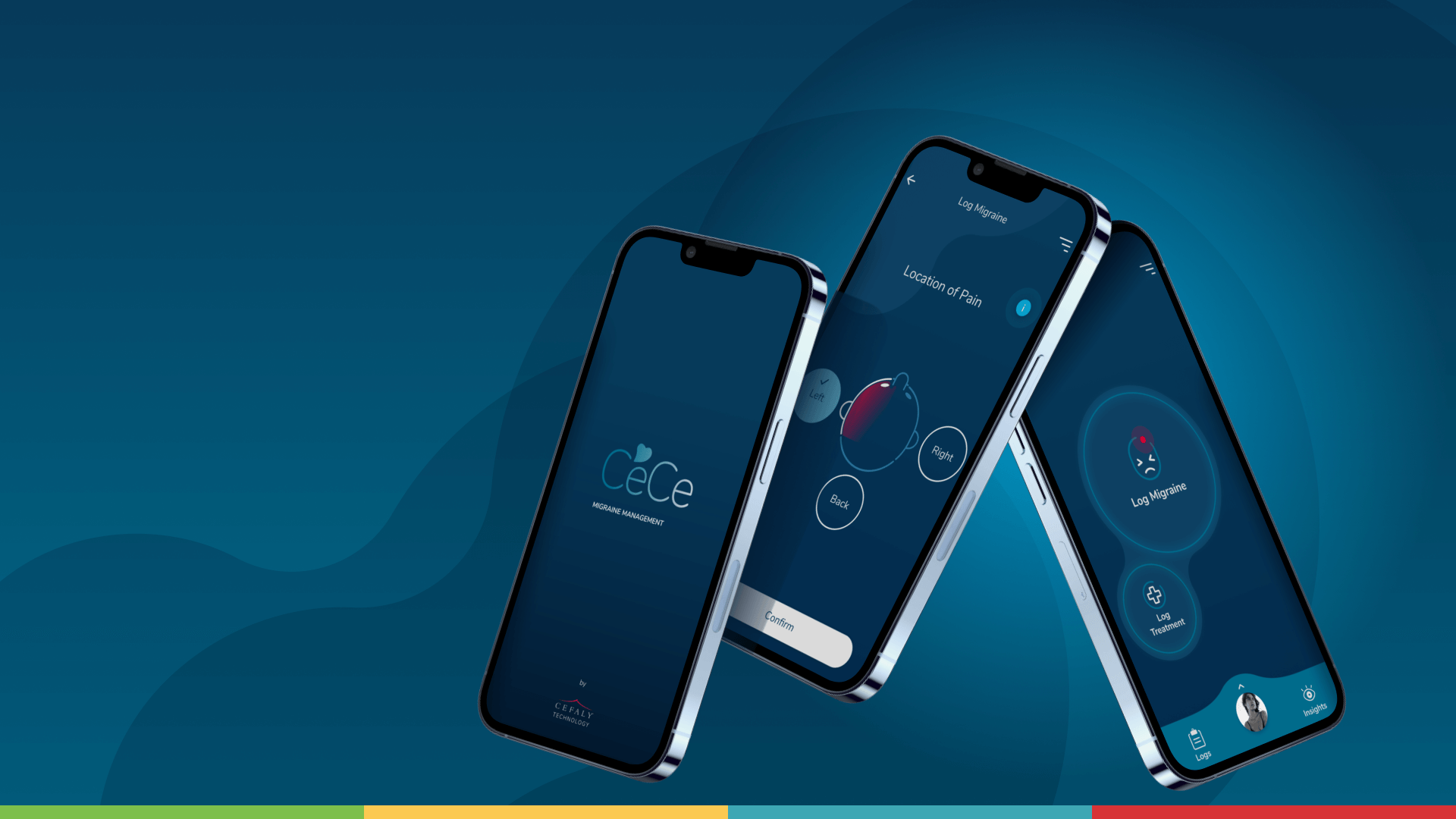
- Super easy onboarding process (via chat) to create a customized migraine profile;
- Real-time logging of migraine attacks and treatments;
- A personalized interface that simplifies migraine tracking;
- Customized insights, such as trends in triggers and timing;
- Optional reminder features that help users stick to their treatment plan.
To meet these requirements, a hybrid mobile application was developed that can be installed on both iOS and Android cell phones and is commercially available.
This app has a special feature. It must be prepared to be a medical device in the near future. Therefore, the design, development, and testing processes should comply with the ISO13485 standard for medical device development and consequently with IEC62304 (for software). This can only be done comfortably by certified companies such as LOAD.
Skills &
Technologies