You
Biorithm
Sector
e-Health
MedTech
Mobile apps
Challenge
In 2018, Biorithm challenged LOAD to design and develop a mobile app that would serve as an interface between the mother and the fetal monitor to retrieve and send data to the backend platform. This was our opportunity to continue to grow as a medical device manufacturer in the e-health sector, allowing our team to create a solution that would be so useful for mothers-to-be. Essentially, the app needed to provide the following flow:
- Patient Registration
The physician registers the patient on the platform.
The physician schedules a virtual care plan.
- Mom Kit
The patient receives the Femom monitor, and also any needed Point of Care devices, such as BP monitors and glucometers.
The patient downloads the Patient mobile app.
- Virtual Visit
The patient completes virtual visits according to a predefined schedule (day/time)
The patient enters their physiological data and symptoms into the Patient app.
- Notification & Review
Automatically classify the patient based on thresholds.
Confirm results and send receipts to patients if needed.
Our approach
To simplify such a complex solution, we list the most important guidelines for the mobile app, so you can better understand what we had to consider during the development phase:
- Generic layout (splash, menu, workspace);
- Login page, authentication, and password recovery;
- Home page with general information/instructions;
- Educational content;
- Patient questionnaire;
- Area with data coming from the Femom device via Bluetooth, with the possibility to enter/modify it manually and send it to the server;
- Visualization area with results;
- A chat area for communication between the doctor and the user;
- Calendar with appointment management;
- Alarms and notifications area;
- Labeling area (related to Medical Device CE Marking requirements).
To meet these requirements, an Android mobile application was developed that has a special feature – it would have to be a medical device with fetal monitor classification. This would mean that the design, development, and testing processes would have to comply with the ISO 13485 standard for medical device development and consequently with IEC 62304 (for software) and ISO 14971 (related to risk management).
This can only be done by certified companies such as LOAD.
Skills &
Technologies
Results
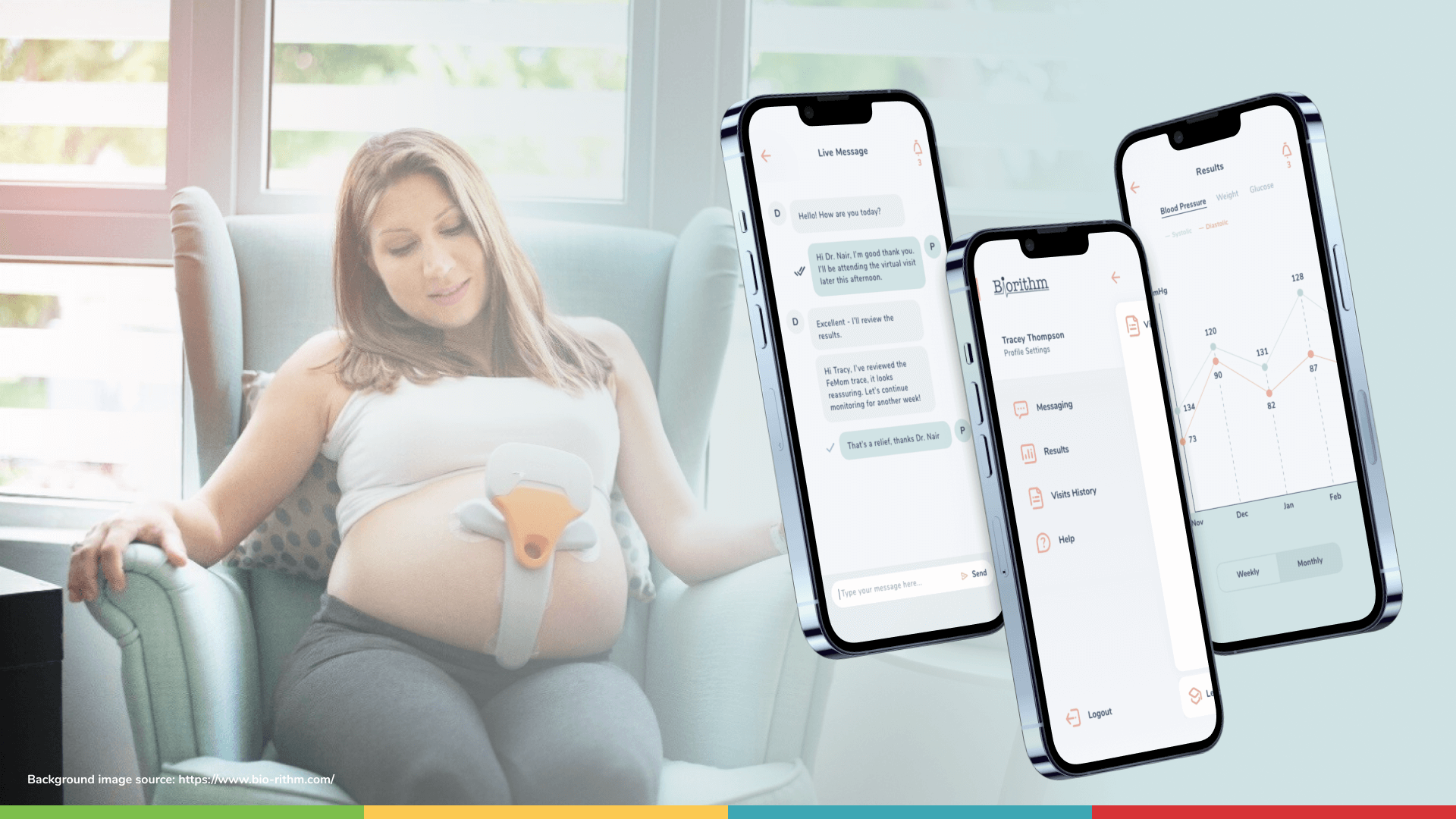
This is how Femom came into being. Femom is a comprehensive remote obstetric monitoring solution that includes:
- A clinical-grade fetal monitor;
- A mobile application with customizable questionnaires;
- Connected Point of Care devices, such as BP monitors and glucometers;
- A dashboard for medical professionals.
The connected platform provides clinicians with new insights into maternal and child health and offers expectant mothers better access to healthcare, more convenience, and more information about their pregnancy.
As agreed with Biorithm, the first version of the app was developed by our team. In the meantime, Biorithm set up its own development team, which later received the source code, documentation, and necessary training and continued the app development on its own.
This was another successful partnership with a medical device company, and the solution is currently on the market.
As a SaMD-certified company, we are very confident that we can slowly but surely shape the future of medicine by delivering digital products that are fully customized to our customer’s needs. Contact us for more information.